A potential drug compound to treat seizures and strokes, researched by Cold Spring Harbor Laboratory and Emory University School of Medicine, went to phase one of clinical trial on April 12.
The trial will be performed by NeurOp, a Georgia biotechnology company, that was co-founded in 2002 by Dr. Dennis Liotta, Executive Director of the Emory Institute for Drug Development at Emory University and Dr. Stephan F. Traynelis, Professor of Pharmacology and researcher at Emory University School of Medicine.
The trial is based off research, collaborated on by Emory University School of Medicine and Cold Spring Harbor Laboratory, that was published in the U.S National Library of Medicine National Institute of Health’s PubMed database in January.
“You want to specifically target effected molecules with small compounds, which we call drugs,” Dr. Hiro Furukawa, leading neuroscientist for Cold Spring Harbor Laboratory’s NMDA research, said.
Causes of stroke include blood clots, the narrowing of an artery, and rupturing aneurysms in the brain, as well as head injuries that cause hemorrhaging.
“Look at a stroke as an attack to the brain,” Joanne Lauten, MSN, SCRN at John T. Mather Hospital Stroke Center in Port Jefferson, said. “When you have a lack of blood flow that area dies off. That’s what causes the damage”.

Traynelis and Liotta have been key developers in researching compounds that target the N-methyl-D-aspartate receptors (NMDA), which is afflicted by stroke. These receptors control memory function. During a stroke, the NMDA receptors overfire, sending an abundance of amino acids, called glutamate, to the area. This overexcitement of neurons is acidic leading to tissue damage which affects brain functions, like motor activity and memory.
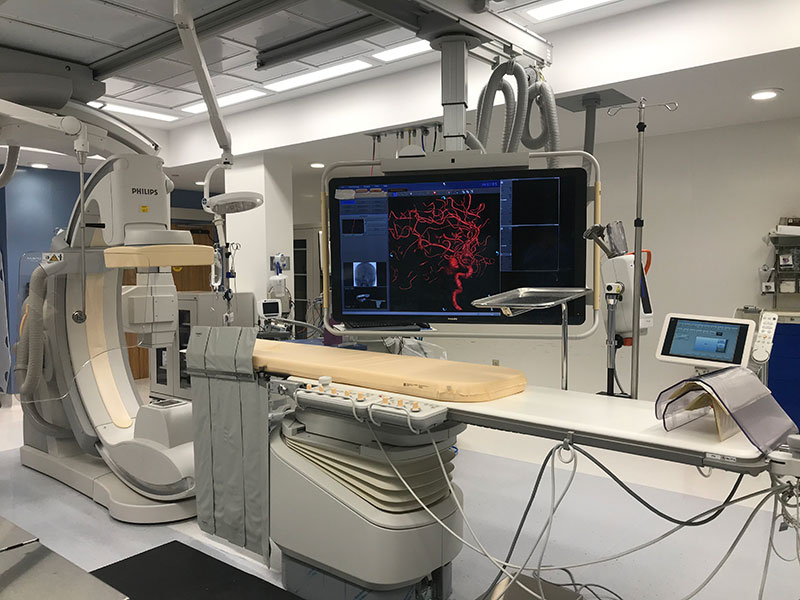
Researchers are looking into the “holy grail of stroke treatment”, Dr. Kimon Bekelis, Director of The Stroke and Brain Aneurysm Center at Good Samaritan Hospital in West Islip, said. The center specializes in treating strokes caused by blood clots in the brain, which they examine using a computerized tomography (CT) scanner.
“The first line of defense in post-stroke treatment is tPA (Tissue Plasminogen Activator), a clot-blocking treatment, which must be administered in the first four hours after suffering a stroke”, Dr. Jason Wallen, lead technician at The Stroke and Brain Aneurysm Center, said.
The window of opportunity can extend all the way up until twenty-four hours after initial symptoms occur, in which surgeons can proceed with a more intensive treatment, of putting a catheter through an insertion in the groin that reaches all the way to the brain to remove the clot.
Stroke is the fifth leading cause of death and the leading cause of long-term disability, according to the Centers for Disease Control and Prevention.
Some patients realize they are having a stroke right away, Lauten said. Others, have what professionals call “slow-progressing stroke,” which can be subtle, like drooling or the sensation that your hand has fallen asleep.
When a clot forms it cuts off a particular vessel “similar to a traffic jam,” Bekelis said. This traffic jam prevents blood from flowing which deprives that area of the brain from oxygen.
“Cars will use different side streets to get to the same destination,” he said. In other words, other blood vessels will distribute blood to the damaged area while the main vessel is blocked up.
The two labs figured out how to isolate the NMDA specifically without damaging surrounding normal regions of the brain. They discovered the 93-series compound has a high specificity level, meaning it more accurately targets one part of the brain without affecting the others.
In 2014, they discovered a pocket in the NMDA receptor that this particular compound binds to, which gave way to chemically testing the theory and proving it. The compound fits into the pocket like an arm in a socket, Furukawa explained.
“This got us excited that, okay, there’s some more tunnels to this thing,” Traynelis said.
During a stroke, the glutamate that is released into the area of the brain is acidic, with causes our brain PH level to dip below its natural state, Furukawa said.
“Our goal was to create compounds that are active at low PH in acidic conditions but inactive normally,” Traynelis said. They found that the 93-series compounds made the receptor neuroprotective, which protects the neuron and reduces the damage of acidification.
Five years ago, Furukawa and his team were able to accurately coordinate the receptor on an x-ray crystallizer, which record data about the molecular structure of a crystal. This led to the first three dimensional model being developed.
“For a chemist to come up with a compound like that, we need information like this,” Furukawa said, while holding up the 3-D model. It takes both biologists and chemists.
“The three-dimensional structural information allows for you to redesign a chemical compound in a way that more specifically binds to that target, which is causing the disease”.
What started as a list of 350 variations of this compound was reduced to fifteen by Furukawa’s lab, which will be tested in NeurOp’s clinical trial.